The 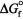 of atomic oxygen is 230.1 kJ/mol.Determine the equilibrium constant for the following reaction at standard thermodynamic conditions: O2(g) 2 O(g) .
of atomic oxygen is 230.1 kJ/mol.Determine the equilibrium constant for the following reaction at standard thermodynamic conditions: O2(g) 2 O(g) .
Definitions:
Test Statistic
A standardized value that is calculated from sample data during a hypothesis test. It is used to determine whether to reject the null hypothesis.
Null Hypotheses
A statement that assumes there is no significant difference or effect in a population, and any observed difference is due to sampling or experimental error.
p-value
A measure used in statistical hypothesis testing to determine the significance of the observed data under a specific hypothesis, often testing if an effect exists.
Standard Deviation
A technique for quantifying the degree of variation or gap among a batch of numbers.
Q12: The reaction,Cr(NH<sub>3</sub>)<sub>6</sub><sup>3+</sup>(aq)+ 3<sup> </sup>en(aq) <span class="ql-formula"
Q21: When pure water autoionizes,which species are produced?<br>A)O<sup>2</sup><sup>-</sup>,OH<sup>-</sup>,H<sub>3</sub>O<sup>+</sup>,and
Q47: Indicate which aqueous solution has the fastest
Q90: You must use 168 g of
Q104: The concentration unit of molality is symbolized
Q111: Consider the following equilibrium: CH<sub>4</sub>(g)+ 2
Q125: What is the overall standard free-energy
Q151: Gaseous carbon monoxide and hydrogen gas
Q179: A proposed mechanism for the reduction
Q210: Consider the following aqueous equilibrium for