Multiple Choice
Using the thermodynamic data below,determine the equilibrium constant for the conversion of oxygen to ozone,3 O2(g) 2 O3(g) ,at 2.00 103 K. 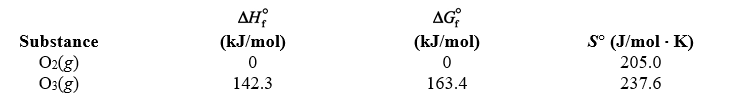
Articulate the significance of cultural and religious assimilation and resistance, particularly relating to the experiences of Native Americans and African slaves.
Explain the dynamics of colonial and native relationships, including alliances, conflicts, and the consequences of European colonization on indigenous societies.
Understand the causes and effects of Bacon's Rebellion and its impact on colonial America.
Analyze the influence of Puritan religious ideas on the governance of the Massachusetts Bay Colony.
Definitions:
Related Questions
Q10: Carbon atoms can be found in a
Q23: Define the term Lewis acid and provide
Q60: The symbol <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6561/.jpg" alt=" The
Q80: Consider the equilibrium,2 NOCl(g) <span class="ql-formula"
Q99: You decide to use your chemistry knowledge
Q103: The d<sub>xy</sub>,d<sub>xz</sub>,and d<sub>yz</sub> orbitals are lower in
Q106: What is the average reaction rate
Q168: If the average rate of reaction
Q208: K<sub>sp</sub> for silver sulfate is 1.2
Q210: Nitrogen dioxide undergoes thermal decomposition according