Using the thermodynamic data below,determine the equilibrium constant for the syngas reaction, CH4(g) + H2O(g) CO(g) + 3 H2(g) ,at 1250 K.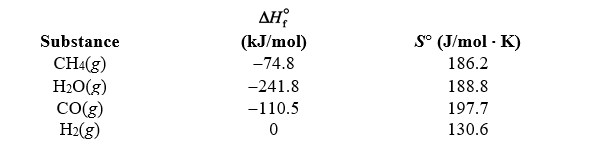
Definitions:
Entry Fee
A charge that must be paid to gain access to a particular service, activity, or location.
Consumption
Refers to the act of using goods and services to satisfy needs and wants.
Income
The money received on a regular basis for work or through investments.
Optimal Consumption
The level of consumption at which a consumer achieves the highest possible satisfaction or utility given their income and the prices of goods and services.
Q31: K<sub>sp</sub> for magnesium fluoride is 6.5
Q38: Which of the following is the
Q47: Complex ions with different ligands have different
Q80: The entropy change in a system
Q86: According to the second law of
Q100: Consider two complexes: (I)[Fe(CN)<sub>6</sub>]<sup>4</sup><sup>-</sup>,and (II)[Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup>.In view of
Q102: Which of the following is a chelation
Q128: The normal temperature range of the
Q145: A ligand is any _ forming a
Q155: For the reaction 4 NH<sub>3</sub>(g)+ 7