Chlorine atoms react with methane,CH4(g)+ Cl(g) HCl(g)+ CH3(g).The rate constant for the reaction is measured at 298,303,308,and 313 K.
(a)Using the plot of ln k vs.1/T,calculate the activation energy and the frequency factor of the reaction.
(b)Estimate the rate constant at 318 K using the Arrhenius equation. 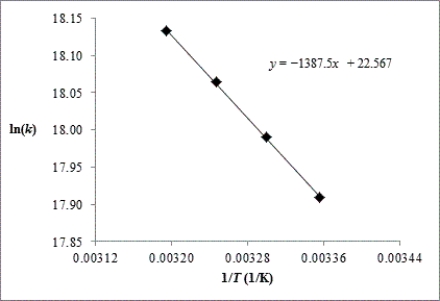
Definitions:
Draw a Single Line Through
A method used in correcting written errors in medical and other records by striking through the mistake with a single line to show the error and the correction clearly.
Continuous Flow
A process operation in which products or materials are produced or moved continuously rather than in distinct batches.
Walk-In/Open Hours Scheduling
A scheduling system that allows patients to receive healthcare services without an appointment during specified times.
Clustering
The process of grouping a set of objects in such a way that objects in the same group (called a cluster) are more similar to each other than to those in other groups.
Q46: In equilibrium expressions,the concentrations of pure solids
Q86: According to the second law of
Q88: Write rate laws for the following
Q108: The following figures represent distributions of gas
Q121: One mole of solid ammonium carbamate (NH<sub>4</sub>CO<sub>2</sub>NH<sub>2</sub>)decomposes
Q133: Use the following data to calculate
Q143: Intravenously administered saline solution must have
Q155: The following graph shows the speed distributions
Q180: Sodium hypochlorite (NaOCl,74.44 g/mol)is a common ingredient
Q189: The linear form of the Arrhenius equation