The energy profiles for four different reactions are shown.Which of the reactions will have the largest rate constant? 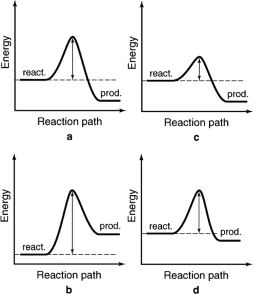
Definitions:
Atm
A unit of pressure defined as exactly equal to 101,325 Pa, representing atmospheric pressure at sea level.
Temperature
A measure of the thermal energy of an object or substance, indicative of how hot or cold it is, typically measured in degrees Celsius, Fahrenheit, or Kelvin.
Helium Gas
A colorless, odorless, tasteless, non-toxic, inert, monatomic gas, the first in the noble gas group in the periodic table. Its boiling and melting points are the lowest among the elements.
Atm
A unit of pressure; standard atmosphere, approximately equal to the Earth's atmospheric pressure at sea level (101.325 kPa).
Q27: A proposed mechanism for the photodecomposition
Q28: Which of the following statements regarding coupled
Q36: Consider the following aqueous equilibrium for
Q48: For the reaction 2 A +
Q53: Indicate which of the following has the
Q58: The following figure shows Arrhenius plots for
Q72: The rate constant for the first-order
Q121: Ethylene glycol is used commonly as
Q127: A chemical equilibrium 2<sub> </sub>A
Q239: What is the pH of a