Multiple Choice
Calculate the maximum amount of work that can be done by the reaction CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) ,given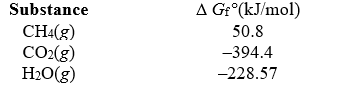
Definitions:
Related Questions
Q10: Cholesterol is poorly soluble in water,but
Q13: Identify the following statement as true or
Q21: How much energy is needed to change
Q84: Hydrofluoric acid,HF,dissociates in water according to
Q103: What is the value of the
Q114: To simulate the pH of blood,which
Q121: Ethylene glycol is used commonly as
Q128: Dinitrogen pentaoxide rapidly decomposes in the
Q131: A gas mixture has partial pressures of
Q151: The enthalpy and entropy of fusion