Hydrogen iodide can theoretically be made by the reaction of hydrogen gas and iodine by the following reaction:
H2(g)+ I2(s) 2 HI(g)
What temperature conditions are required for the formation of HI to be favored at standard pressure?
S (H2,g)= 130.6 J/mol . K
S (I2,s)= 116.1 J/mol . K
S (HI,g)= 206.6 J/mol . K
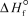 (HI,g)= 26.5 kJ/mol . K
(HI,g)= 26.5 kJ/mol . K
Definitions:
Sender
In communication, the party who initiates the message and transmits it to the receiver.
Decoding
The process by which the receiver interprets the sender’s message.
IMC Message
Integrated Marketing Communications message, which ensures all forms of communications and messages are carefully linked together to provide a unified message across all marketing channels.
Television Commercial
A form of advertising broadcast on television, designed to promote a product, service, or message to a wide audience.
Q22: One compound under investigation for use
Q42: The following figure shows an uncatalyzed reaction
Q50: A sample of hydrogen gas at
Q51: A proposed mechanism for the reaction
Q79: For the equilibrium N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)
Q88: Given equal masses of the following,which will
Q94: An equilibrium that strongly favors products has
Q143: Which of the following statements regarding the
Q144: The entropy of fusion for ice
Q159: Suppose the surface-catalyzed hydrogenation reaction of an