Estimate the free-energy change for the combustion reaction of glucose at body temperature (37 C)using the data given.If the phosphorylation of one mole of ADP3- involves a free energy change of +30.5 kJ,about how many moles of ATP4- could be made from the combustion of one mole of C6H12O6,assuming no energy loss?
Substance 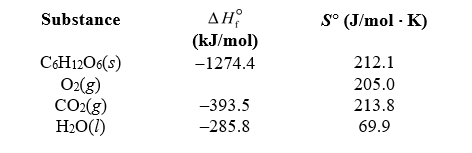
Definitions:
Sociocultural Perspective
An approach in psychology that examines how societal and cultural influences impact human behavior and mental processes.
Customs
Traditional practices or behaviors that are characteristic of a specific society, place, or time, often relating to the conduct of everyday life.
Beliefs
Cognitive representations about the world, oneself, or others, that are accepted by an individual as true or existing.
Evolutionary Psychology
A theoretical approach in the social and natural sciences that examines psychological structure from a modern evolutionary perspective.
Q9: Given the following data,determine the rate
Q45: The observed rate law for the
Q50: What is the change in the
Q51: When can an x be ignored in
Q80: Chlorine atoms react with methane,CH<sub>4</sub>(g)+ Cl(g)
Q120: A solution is prepared by mixing
Q142: What is the average kinetic energy
Q173: During the decomposition of CaCO<sub>3</sub>(s)into CaO(s)and
Q192: The pressure gauge on a cylinder
Q202: Collision theory assumes that the rate of