Create the Born-Haber cycle and calculate the lattice energy of sodium fluoride from the following data: Ionization energy of Na: 496 kJ/mol
Electron affinity of F: -328 kJ/mol
Energy to vaporize Na: 108 kJ/mol
F2 bond energy: 160 kJ/mol
Energy change for the reaction:
Na(s) + 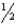 F2(g) NaF(s) ; H = -575 kJ
F2(g) NaF(s) ; H = -575 kJ
Definitions:
Delphi Technique
A method of group communication and decision-making that utilizes a panel of experts who anonymously provide feedback and predictions.
Compromise Settlements
Agreements reached by all parties involved through concessions and adjustments, often used to resolve disputes or conflicts.
Integrative Solutions
Solutions that satisfy the interests of all parties involved in a negotiation, creating a win-win situation.
Team Negotiation
A collaborative process where members of a team work together to negotiate terms or solutions with an opposing party.
Q2: A sample of gas at 475.0<sup>
Q12: The vapor pressure of an aqueous
Q15: Which one of the following is not
Q28: At a given temperature,nitrogen gas effuses 1.194
Q37: You are an industrial engineer in
Q84: Hydrofluoric acid,HF,dissociates in water according to
Q86: A 3.0 <span class="ql-formula" data-value="\times"><span
Q105: A chemist is planning to study
Q107: The isomerization reaction of cyclopropane to
Q113: Isooctane is a good model for gasoline.When