Determine the energy change for the reactio Li(s) + 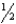 Cl2(g) LiCl(s) from the following data:
Cl2(g) LiCl(s) from the following data:
Lattice energy of LiCl = -861 kJ/mol
Energy to vaporize Li = 159 kJ/mol
Ionization energy of Li = 520 kJ/mol
Cl2 bond energy: 240 kJ/mol
Electron affinity of Cl: -349 kJ/mol
Definitions:
Job Performance
The level of effectiveness and efficiency with which an employee fulfills their assigned roles and tasks.
Orientation
The process of introducing new employees to an organization, its values, team members, and work environment.
Co-workers
Individuals who work together in the same organization or on the same team, often sharing tasks and responsibilities.
Activities
Actions or tasks that individuals or groups undertake as part of their roles or functions within an organization.
Q7: The energy profiles for four different reactions
Q29: The standard molar enthalpy fusion for
Q42: What is the boiling point of
Q57: Describe how you would use the data
Q77: The cold inflation pressure of many
Q80: Which of the following statements regarding the
Q96: A solution contains 6.50 mol water,0.300
Q108: Acetone is a fairly volatile liquid
Q124: Which of the following statements regarding calorimetry
Q143: The decomposition of NOCl to form