Create the Born-Haber cycle and calculate the lattice energy of sodium fluoride from the following data: Ionization energy of Na: 496 kJ/mol
Electron affinity of F: -328 kJ/mol
Energy to vaporize Na: 108 kJ/mol
F2 bond energy: 160 kJ/mol
Energy change for the reaction:
Na(s) + 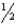 F2(g) NaF(s) ; H = -575 kJ
F2(g) NaF(s) ; H = -575 kJ
Definitions:
Job Characteristics Theory
A theory that suggests jobs can be designed with specific characteristics that enhance employee motivation, satisfaction, and productivity.
Ideal Job
A professional position perceived as perfectly suiting an individual's career aspirations, skills, and personal values.
Procedural Justice
The fairness and transparency of the processes used by authorities to make decisions, often judged based on consistency, bias suppression, accuracy, correctability, and representativeness.
Multiplicative Relationship
A situation or equation in which one variable increases as a result of increasing another variable by a specific factor or multiplier.
Q30: What is the standard entropy change when
Q32: In terms of the enthalpy of formation,which
Q40: A 0.300 g sample of caffeine
Q43: Suppose 3.95% of 521 g PCl<sub>5</sub>
Q47: When carbon monoxide forms a crystal,each
Q82: Place the following gases in order of
Q98: The reaction of NO gas with
Q123: What is the molar mass of a
Q137: For the reaction 2 A +
Q163: What is the density of sulfur