Use the following data to calculate the enthalpy change for the following reaction: K(s) + 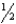 Cl2(g) KCl(s)
Cl2(g) KCl(s) 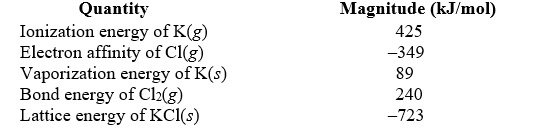
Definitions:
Activity-Based Costing
A pricing approach that discerns various tasks within a business and allocates the expenses of each activity to all goods and services based on their real usage.
Breakeven Point
The point at which total costs and total revenues are equal, meaning a business neither makes a profit nor incurs a loss.
Unit Level
A classification in costing referring to activities or costs that are directly associated with the units of product produced.
Environmental Management Accounting
The management of environmental and cost information to improve business decisions related to environmental performance.
Q4: Which of the following substances,a-d,will release the
Q18: Given the following two measurements of
Q42: Which of the following is a not
Q52: Henry's law constant for oxygen dissolving
Q56: The density of an unknown gas
Q86: A 3.0 <span class="ql-formula" data-value="\times"><span
Q118: Which of the following statements regarding spontaneous
Q123: What is the molar mass of a
Q167: Which of the following does NOT
Q178: The initial rate data for the