Given that molar enthalpy of combustion of acetylene (C2H2,26.04 g/mol) is -1299 kJ/mol at 25°C and 1 atm,which of the following is false? 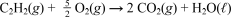
Definitions:
VDRL
A blood test for syphilis that detects antibodies produced against components of damaged cells.
NGU
Non-Gonococcal Urethritis, an inflammation of the urethra not caused by gonorrhea, typically resulting from infection.
Sexual Intercourse
The act involving the physical union and genital contact between individuals, often leading to the exchange of sexual fluids.
Surgical Removal
The process of excising or cutting out a diseased or unwanted part of the body, typically through an operative procedure.
Q3: What is the density of propane
Q6: Which of the following compounds is capable
Q34: In an experiment,30.00 g of aluminum at
Q38: A 75.0 mg sample of a
Q45: Determine the root-mean-square speed of methane
Q56: Dispersion forces are due to _<br>A)permanent dipoles.<br>B)temporary
Q68: In a Reuters news report dated
Q81: Indicate which of the following molecules exhibits
Q96: Which of the following is unimportant when
Q171: A certain rust remover containing phosphoric acid