Given the following reactions,estimate the overall enthalpy change for dissolution of solid sodium hydroxide in water. NaOH(s) NaOH(aq) 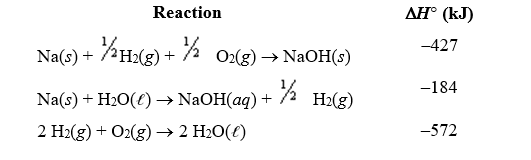
Definitions:
Debit and Credit
Accounting terms used to record transactions in the accounts, where debits increase asset or expense accounts and decrease liability, equity, or revenue accounts, and credits do the opposite.
Operations
The day-to-day activities involved in running a business that lead to the production of goods and delivery of services.
Journalizing
Journalizing is the process of recording transactions in a company's journal prior to posting them to the general ledger.
Posting Process
The procedure of transferring entries from a company's journal into the appropriate accounts in its ledger, which helps in summarizing financial activities.
Q20: A sample of oxygen gas at
Q21: Aqueous potassium phosphate is added to 545
Q26: Han purple,a synthetic inorganic pigment developed in
Q40: Carbon emissions by China in 2012
Q42: Indicate which of the following compounds will
Q45: Sulfuric acid reacts with a vanadium
Q86: A 3.0 <span class="ql-formula" data-value="\times"><span
Q112: What is the root-mean-square speed of
Q123: Ammonia (NH<sub>3</sub>,17.04 g/mol)is industrially produced from N<sub>2</sub>
Q207: What mass of silver chloride will be