Given the following reactions,what is the overall enthalpy change for the following reaction? C4H4(g) + 2 H2(g) C4H8(g) 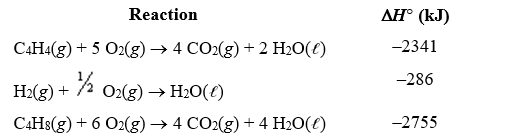
Definitions:
Profit Maximization
The procedure through which a business identifies the pricing and production volume that yields the highest earnings.
Market Price
The price at which a good or service is offered in the marketplace.
Profit Maximization
A rephrased definition: The strategy or aim of a firm to achieve the highest profit possible, usually by adjusting outputs, prices, or production costs.
Market Price
The current price at which an asset or service can be bought or sold in the open market.
Q25: Suppose the brain needs to metabolize
Q27: Which of the following substances will require
Q39: What is the osmotic pressure of
Q60: How many mL of 0.116 M Ba(OH)<sub>2</sub>
Q62: The surroundings perform work on a
Q103: How many moles of solute are there
Q104: The concentration unit of molality is symbolized
Q128: The normal temperature range of the
Q164: There is concern that the combustion of
Q198: Ammonium nitrate is an ingredient in