Determine the standard enthalpy of formation for NO given the following information about the formation of NO2 under standard conditions,and 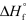 (NO2) = + 33.2 kJ/mol.
(NO2) = + 33.2 kJ/mol.
2 NO(g) + O2(g) 2 NO2(g) Hrxn = -114.2 kJ
Definitions:
Filtering
The process of modifying or withholding information to manage a person’s reactions.
Active Listeners
individuals who pay close attention to the speaker, engage in feedback, and retain the information being communicated.
Components of Listening
Elements essential for effective listening, including hearing, understanding, evaluating, remembering, and responding.
Management by Walking Around
A leadership approach where managers informally walk around the office to check on the work status of employees, engage in conversations, and foster a more open and accessible organizational environment.
Q8: Silver salts are used in black-and-white
Q17: Na<sub>2</sub>B<sub>4</sub>O<sub>5</sub>(OH)<sub>4 </sub><sup> </sup>.<sup> </sup>8 H<sub>2</sub>O,commonly known as
Q33: One form of elemental sulfur is a
Q53: Metallic copper can be obtained from the
Q84: Calculate the molality of a solution
Q105: A typical adult body contains about 5.0
Q116: Write the formula and calculate the mass
Q132: Hydrogen gas (2.02 g/mol)can be produced
Q167: Which of the following does NOT
Q179: Imagine a sample of water vapor at