Given that molar enthalpy of combustion of acetylene (C2H2,26.04 g/mol) is -1299 kJ/mol at 25°C and 1 atm,which of the following is false? 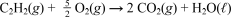
Definitions:
Deadline
A set date or time by which something must be completed or submitted, often used to ensure timely completion of tasks.
Clustering
A method of organizing ideas by grouping similar items, concepts, or themes together.
Gathering
The process of collecting information or data from various sources for a specific purpose.
Drafts and Revisions
The process of creating preliminary versions of documents or designs, followed by refining and improving them based on feedback or reflection.
Q18: How does the presence of solutes raise
Q36: Gasoline is primarily a mixture of
Q38: Nitric oxide (30.01 g/mol)can be produced
Q49: Baking soda (NaHCO<sub>3</sub>,84.0 g/mol)can react with
Q57: If a mass of 100.0 g moves
Q81: Indicate which of the following molecules exhibits
Q92: Air,which is primarily nitrogen and oxygen,does
Q95: Which molecules in air have the highest
Q99: What would be the freezing point
Q127: Write the net ionic equation for