Given the following reactions,what is the overall enthalpy change for the following reaction? C4H4(g) + 2 H2(g) C4H8(g) 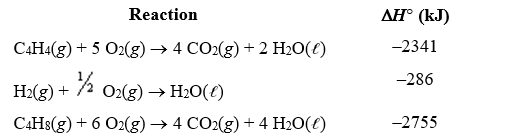
Definitions:
Process Costing Systems
Process costing systems are accounting methods used to allocate production costs to units of output, particularly effective in industries that produce identical or similar products in continuous processes.
FIFO Method
An inventory valuation method that assumes the first items placed into inventory are the first sold, standing for "First-In, First-Out."
Work in Process Inventory
Goods and materials that are partially completed in the manufacturing process but not yet ready for sale.
FIFO Method
A inventory valuation method that assumes the first items purchased or produced are the first ones sold, standing for "First In, First Out."
Q11: Given the standard enthalpies of formation for
Q36: Which of the following is an endothermic
Q79: Lead levels in drinking water should
Q86: A 3.0 <span class="ql-formula" data-value="\times"><span
Q117: Which of the following samples of an
Q117: Potassium is a very reactive metal,but in
Q129: What is the boiling point of
Q147: Given 150 grams each of water and
Q161: Which of the following is a weak
Q161: How high (in feet)can one atmosphere of