Determine the standard enthalpy of formation for NO given the following information about the formation of NO2 under standard conditions,and 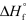 (NO2) = + 33.2 kJ/mol.
(NO2) = + 33.2 kJ/mol.
2 NO(g) + O2(g) 2 NO2(g) Hrxn = -114.2 kJ
Definitions:
Marginal Products
Marginal Product is the extra output, or product, produced by using one more unit of a production input, holding all other inputs constant.
Total Product
The total quantity of output produced by a firm over a given period.
Dogs Washed
The service or action of cleaning dogs by bathing them, often as a professional service.
Diminishing Returns
A principle stating that if one factor of production is increased while others are held constant, the incremental increases in output will eventually decrease.
Q5: A burner in a gas grill mixes
Q25: Suppose the brain needs to metabolize
Q28: Which statement about the following chemical
Q64: A mixture of salt and ice
Q84: The standard enthalpy of combustion of propane
Q87: Which statement,A-D,regarding photosynthesis is NOT correct? In
Q126: Create the Born-Haber cycle and calculate the
Q188: Identify the base in the following
Q192: The pressure gauge on a cylinder
Q210: What volume of 3.00 M NaOH (40.0