Coal is converted into cleaner,more transportable fuels by burning it with oxygen to produce carbon monoxide.The carbon monoxide then is reacted with hydrogen using a catalyst to produce methane and water.Is the reaction between CO and H2 exothermic or endothermic,and what is the change in enthalpy for it? The enthalpies of formation of the reactants and products are given below. 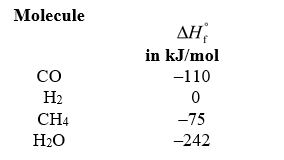
Definitions:
Q3: Which one of the ionic compounds below
Q33: A sample of propane gas is contained
Q54: How many moles of an ideal
Q65: The dipole moments of HCl and of
Q68: Nitrous oxide (N<sub>2</sub>O,44.02 g/mol)has been used
Q123: What are the products in the
Q130: Which of these gases (Ar,N<sub>2</sub>O,H<sub>2</sub>)has the
Q137: What are the signs of
Q153: The van der Waals constants for
Q182: Diluting 1.0 mL of a 1.0