Use the bond energies in the table below to estimate the enthalpy change associated with the hydrogenation reaction of acetylene.
HC  CH(g) + 2 H2(g) CH3CH3(g)
CH(g) + 2 H2(g) CH3CH3(g) 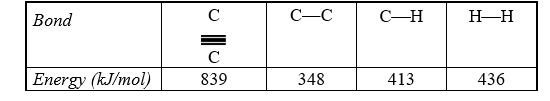
Definitions:
Environmental Economists
Experts who study the economic impacts of environmental policies and the cost-benefit analyses of preserving or degrading the environment.
Neoclassical Economics
An economic theory that focuses on supply, demand, and the price mechanism as the driving forces behind production, distribution, and consumption of goods and services.
Economic Systems
Organized ways in which countries manage their resources and distribute goods and services within their societies.
Principles
Fundamental truths or propositions that serve as the foundation for a system of belief or behavior or for a chain of reasoning.
Q5: Which is the total ionic equation
Q17: Na<sub>2</sub>B<sub>4</sub>O<sub>5</sub>(OH)<sub>4 </sub><sup> </sup>.<sup> </sup>8 H<sub>2</sub>O,commonly known as
Q22: One compound under investigation for use
Q62: Hydrogen iodide can theoretically be made
Q72: Determine the normal melting point of benzoic
Q73: When the oxidation-reduction reaction shown here
Q74: Indicate which aqueous solution has the lowest
Q83: Combustion analysis of an organic compound to
Q93: When the oxidation-reduction reaction shown here
Q172: In carrying out a titration of a