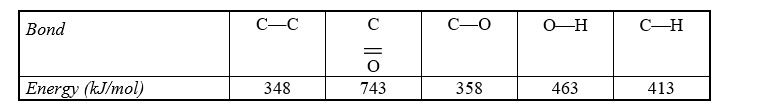
Use the bond energies in the table below to estimate the enthalpy change associated with the reaction of acetic acid with ethanol to make ethyl acetate. 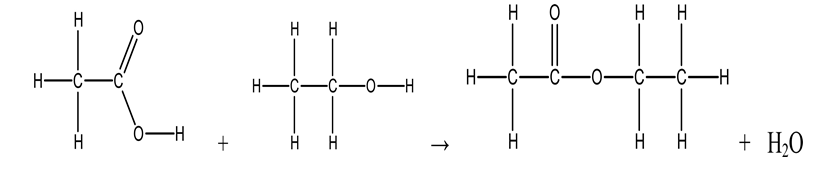
Definitions:
Accrued Revenue
Income earned but not yet received or recorded at the end of the accounting period.
Shareholders' Equity
The residual interest in the assets of a company after deducting its liabilities, representing the ownership stake of the shareholders.
Net Income
The total profit or loss of a business after all expenses, including taxes and interest, have been subtracted from total revenue.
Deferred Revenue
Money received by a company for goods or services which have not yet been delivered or provided, recognized as a liability.
Q23: What is the freezing point of
Q41: Acrylonitrile (53.07 g/mol)can be produced from
Q47: A sample of oxygen gas placed
Q82: Which of the following should have the
Q89: Polymers with ionic functional groups have been
Q90: Nitrogen-fixing bacteria and plants are capable of
Q128: The volume change associated with a
Q178: A 25.00 mL standard adult dose of
Q197: In a titration,the solution of known concentration
Q203: How many grams of nickel(II)phosphate (366