In an aqueous solution under basic conditions,ozone (O3) reacts with iodide (I-) and water to form iodine (I2) ,hydroxide (OH-) ,and oxygen (O2) .What is the sum of the stoichiometric coefficients in the balanced reaction equation? O3(g) + I-(aq) + H2O 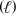 I2(aq) + OH-(aq) + O2(g)
I2(aq) + OH-(aq) + O2(g)
Definitions:
Price Elasticity
A measure indicating the degree to which product demand is affected by price shifts.
Quantity Supplied
The amount of a good or service that producers are willing and able to sell at a given price over a specified period.
Price Increase
A Price Increase refers to a rise in the cost of goods or services that can occur due to various factors like inflation, increased production costs, or higher demand.
Immediate Market Period
A very short time frame in economics during which the supply of a good is completely fixed and cannot respond to changes in demand.
Q19: Hard water,which contains Mg<sup>2+</sup> and Ca<sup>2+</sup> ions,tends
Q31: Which liquid,water or ethanol,would you expect to
Q37: You are an industrial engineer in
Q46: The molar heat capacities of zinc,titanium,and iron
Q69: Determine the bond order of S<sub>2</sub><sup>-</sup>.<br>A)3<br>B)2.5<br>C)2<br>D)1.5<br>E)1
Q80: What is the geometry of the ClF<sub>4</sub><sup>-</sup>
Q114: Balance the following redox reaction under
Q139: How many shared electron pairs are there
Q143: In a demonstration of the complete combustion
Q149: The proper name for Cr<sub>2</sub>S<sub>3</sub> is _<br>A)chromium(III)sulfide.<br>B)chromium(II)trisulfide.<br>C)chromium