Potassium superoxide (KO2,71.10 g/mol) can be used to generate oxygen gas.What is the theoretical yield of O2 (32.00 g/mol) if 500.0 g KO2 reacts with excess H2O (18.02 g/mol) ? 4 KO2(s) + 2 H2O 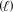 4 KOH(s) + 3 O2(g)
4 KOH(s) + 3 O2(g)
Definitions:
Interest Rates
The proportion of an amount of money levied for its utilization, denoting the expense of borrowing funds or the profit on investments.
Market Value
The prevailing cost at which an asset or service can be traded or acquired.
Face Value
The nominal or dollar value printed on a financial instrument like a bond or stock certificate.
Premium
An amount paid for an insurance policy or an amount above the nominal value of a security or investment.
Q3: How many moles of nitrate ions are
Q54: The reaction of nitrogen with oxygen
Q66: Coulomb's law states that the interaction energy
Q99: One reason electronegativity increases from left to
Q104: The relative energies (strengths)of the intermolecular forces
Q114: Balance the following redox reaction under
Q136: Not all the bond vibrations of carbon
Q139: Which statement about the following chemical
Q141: Write the net ionic equation for
Q148: From year to year,the water level in