Acrylonitrile (53.07 g/mol)can be produced from propylene (42.09 g/mol)according to the reaction shown below.If the percent yield of the reaction is only 45.7%,how many grams of C3H6 should be used if 1250 g C3H3N must actually be produced?
4 C3H6(g)+ 6 NO(g) 4 C3H3N 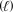 + 6 H2O
+ 6 H2O 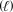 + N2(g)
+ N2(g)
Definitions:
Cost Of Goods Sold
The amount of money a firm spent to buy or produce the products it sold during the period to which the income statement applies.
Q30: Which picture best represents an atomic-level view
Q44: In an experiment,1.50 kg of concrete at
Q44: A substance that is _ will be
Q96: Draw the Lewis structures of PCl<sub>3</sub> and
Q101: Identify the hybridization of atomic orbitals for
Q103: Calcium carbide (CaC<sub>2,</sub> 64.10 g/mol)reacts with water
Q122: Commercial hydrochloric acid is 12.1 M.What volume
Q125: How many moles of ammonia are there
Q133: Ethanol has the formula CH<sub>3</sub>CH<sub>2</sub>OH.The end carbon
Q198: Ammonium nitrate is an ingredient in