In an aqueous solution under basic conditions,ozone (O3) reacts with iodide (I-) and water to form iodine (I2) ,hydroxide (OH-) ,and oxygen (O2) .What is the sum of the stoichiometric coefficients in the balanced reaction equation? O3(g) + I-(aq) + H2O 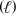 I2(aq) + OH-(aq) + O2(g)
I2(aq) + OH-(aq) + O2(g)
Definitions:
Exchange Rate
The price of one country's currency expressed in another country's currency, facilitating international trade and finance.
Argentine Peso
The official currency of Argentina, utilized in financial transactions within the country.
American Truck
A vehicle designed and manufactured typically in the United States for the purpose of transporting goods and materials.
Exchange Rate
The value of one currency expressed in terms of another currency, used when converting from one currency to another.
Q3: How many moles of nitrate ions are
Q7: Which of the following regarding state and
Q41: Active metals often form a protective oxide
Q54: How many moles of an ideal
Q56: Nitrogen and oxygen can react to form
Q57: Ethylamine,C<sub>2</sub>H<sub>5</sub>NH<sub>2</sub>,acts as a weak base in
Q60: Ammonia,NH<sub>3</sub>,is industrially produced from N<sub>2</sub> and H<sub>2</sub>
Q94: What is the steric number of the
Q145: The electron group geometry of AsH<sub>3</sub> is
Q148: From year to year,the water level in