Acrylonitrile (53.07 g/mol)can be produced from propylene (42.09 g/mol)according to the reaction shown below.If the percent yield of the reaction is only 45.7%,how many grams of C3H6 should be used if 1250 g C3H3N must actually be produced?
4 C3H6(g)+ 6 NO(g) 4 C3H3N 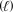 + 6 H2O
+ 6 H2O 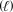 + N2(g)
+ N2(g)
Definitions:
Water-Diamond Paradox
An economic paradox that questions why diamonds are pricier than water despite water being essential for survival and diamonds having relatively little practical use.
Marginal Utility
The extra pleasure or benefit a consumer receives from using an additional unit of a product or service.
Demand Schedule
A table that lists the quantity of a good that consumers are willing and able to purchase at varying price points, at a specific moment in time.
Total Utility
The total satisfaction or benefit that a consumer derives from consuming a certain quantity of goods or services.
Q12: Oxygen has two common molecular anions: peroxide
Q26: In demonstrations of strong and weak electrolytes
Q28: Which of the following statements regarding bonding
Q61: Viscosity is a measure of a substance's
Q87: What is the oxidizing agent in
Q88: What are the stoichiometric coefficients for oxygen
Q97: Identify the base in the following
Q122: Describe the valence bond picture of
Q162: In comparing the Fraunhofer lines with light
Q189: If there are 0.505 g of NaCl