A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below.Taking into account the nonbonding electrons,identify the hybridization of the atomic orbitals for the following atoms: O1,O2,and O3.Identify the bond angles around C1,C2,and O3. 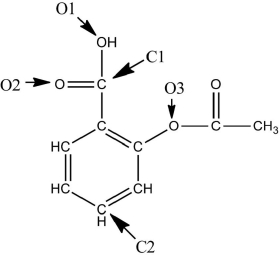
Definitions:
Probability Values
Numerical values that represent the likelihood of the occurrence of a specific event or outcome.
Mutually Exclusive
Two or more events that cannot happen at the same time; the occurrence of one event precludes the occurrence of the other(s).
Nonzero Probabilities
Probabilities that are greater than zero, indicating that an event has a chance of occurring.
Sample Space
The set of all possible outcomes or results in an experiment or a random trial.
Q19: In a chemical reaction,bonds are broken and
Q20: You are given a liquid sample that
Q37: How many covalent bonds are there in
Q42: Indicate which of the following compounds will
Q74: Which of the following oxides of nitrogen
Q76: Assuming complete combustion,how many grams of butane
Q95: Sodium thiosulfate (Na<sub>2</sub>S<sub>2</sub>O<sub>3</sub>,molar mass = 158.2
Q102: What is the formula for sodium bromide?<br>A)SBr<br>B)NaBr<br>C)Na<sub>2</sub>Br<br>D)NaBrO<br>E)NaBr<sub>2</sub>
Q107: Use the formal charge as a criterion
Q115: Which of the following lasers emits