A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below.Taking into account the nonbonding electrons,identify the hybridization of the atomic orbitals for the following atoms: O1,O2,and O3.Identify the bond angles around C1,C2,and O3. 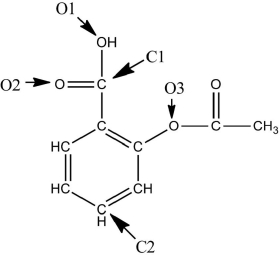
Definitions:
Showrooming Problem
A retail challenge where customers browse products in a physical store only to buy them later online at a lower price, affecting the store's sales.
Pull Model
A supply chain management strategy where production is based on demand, with products pulled through the supply chain by consumer demand.
Customer Order
A request made by a client to purchase goods or services from a business, initiating a transactional process.
Fast Fashion
A term used to describe clothing designs that move quickly from the catwalk to stores to meet new trends.
Q4: The combustion of butane (C<sub>4</sub>H<sub>10</sub>)forms carbon dioxide
Q7: Atomic spectra are due to the changes
Q50: Which of the following compounds would be
Q62: The amide functional group is the fundamental
Q62: For each of the elements below,there are
Q80: What is the geometry of the ClF<sub>4</sub><sup>-</sup>
Q84: Draw the Lewis structure for the nitrate
Q135: The average atomic mass of nickel is
Q137: In quantum mechanics,an atomic orbital _<br>A)provides the
Q142: Which statement is true about the mole