Which of the following statements is false regarding the Lewis structure of trichloroacetic acid,CH2Cl3O2? A partial bonding framework is given. 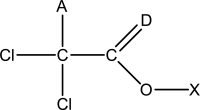
Definitions:
DSM-5
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, a comprehensive classification system for psychiatric disorders.
Real-World Tests
Practical evaluations conducted under natural or operational conditions as opposed to controlled or laboratory conditions to assess the performance of a system or individual.
Reliability
Refers to the consistency of a research study or measuring test.
Major Depressive Disorder
An emotional disorder characterized by never-ending depressive feelings or a disinterest in normal activities, significantly obstructing day-to-day life.
Q7: The resistance of a liquid to an
Q7: Atomic spectra are due to the changes
Q8: Cadaverine is composed of carbon,hydrogen,and nitrogen.A 20.000
Q13: Fe<sub>2</sub>O<sub>3</sub>(s)and powdered aluminum can react with great
Q45: Three 2p<sub>z</sub> orbitals on three oxygen
Q54: Fraunhofer lines are due to _<br>A)emission of
Q71: What is the total number of electrons
Q107: Which of the following statements about the
Q129: What <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6561/.jpg" alt="What quantum
Q145: What information do boundary-surface representations of orbitals