Estimate the enthalpy of reaction for 2 H2(g) + O2(g) 2 H2O(g) using bond energies.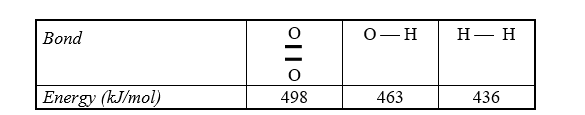
Definitions:
Osteoporosis
A condition characterized by the weakening of bones, making them fragile and more likely to break.
Complete Protein
A source of protein that contains an adequate proportion of all nine essential amino acids necessary for dietary needs.
Dietary Intake
The range and amount of food and drink consumed by an individual or group.
Vegetables
Edible plants or parts of plants, typically consumed for their nutritional value.
Q11: What is the hybridization of arsenic in
Q18: What is the theoretical yield of
Q21: Which one of the following Lewis symbols
Q23: Angelic acid is 59.98% C,8.05% H,and 31.96%
Q38: Nitric oxide (30.01 g/mol)can be produced
Q40: For the molecule CH<sub>3</sub>CHCHCH<sub>3</sub>,the local molecular geometry
Q81: Draw the Lewis structure of BrF<sub>5</sub>.Give the
Q123: Which of the following statements is NOT
Q132: Which of the following represents the
Q173: Which molecule has a stretching vibration that