Estimate the enthalpy of reaction for 2 CO(g) + O2(g) 2 CO2(g) using bond energies. 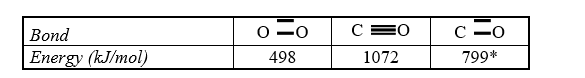
* C  O bond in CO2
O bond in CO2
Definitions:
Multidisciplinary Crisis Response Team
A group composed of professionals from various fields who work together to address and manage crisis situations efficiently and effectively.
Media Liaison
The person or role responsible for managing communication and interactions between an organization and the media.
Trauma
A deeply distressing or disturbing experience, often resulting in lasting psychological impact.
Mental
Relating to the mind, cognitive processes, or psychological state of an individual.
Q5: How many nonbonding electrons are there in
Q37: How many covalent bonds are there in
Q62: Vinegar is a solution of acetic acid
Q75: Write the complete atomic symbol with both
Q94: The emission spectra of Na and Na<sup>+</sup>
Q97: How many unpaired electrons does the nitride
Q104: De Broglie reasoned that for the electron
Q157: What types of bonds form between the
Q163: The first ionization energies of phosphorus and
Q169: Cesium has a work function of