The energy change for an electronic transition in a one-electron atom or ion (H,He+,Li2+,etc.) from ninitial to nfinal is given by 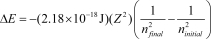 ,where Z is the atomic number. Which one of the following species will have the longest wavelength emission line for the transition between the ninitial = 2 and nfinal = 1 levels?
,where Z is the atomic number. Which one of the following species will have the longest wavelength emission line for the transition between the ninitial = 2 and nfinal = 1 levels?
Definitions:
Dividend Corporation
A company known for regularly distributing a portion of its earnings to shareholders in the form of dividends.
Stock Price
The cost of purchasing a share of a company in the stock market at any given time.
Sharpe Measure
A metric used to evaluate the risk-adjusted return of an investment portfolio, comparing its excess return to the standard deviation of the portfolio's returns.
Risk-Free Return
The theoretical return on an investment with zero risk of financial loss, typically associated with government bonds.
Q7: What did artist Franz Marc paint because
Q32: Who exhibited with the Surrealists but never
Q60: Indicate which of the following pairs of
Q60: Which of the following is NOT a
Q63: Who discovered electrons?<br>A)Henri Becquerel<br>B)Robert Millikan<br>C)Joseph John Thomson<br>D)John
Q64: What is the energy (E,in J)of
Q91: Rank the following ionic compounds in order
Q103: A strontium-90 atom that has a lost
Q105: Determine the molecular geometry of N<sub>2</sub>O.<br>A)bent<br>B)trigonal planar<br>C)linear<br>D)tetrahedral<br>E)trigonal
Q148: The electron group geometry of GaCl<sub>3</sub> is