Consider the radial distribution shown below for an electron in an s atomic orbital where r is the distance of the electron from the nucleus. 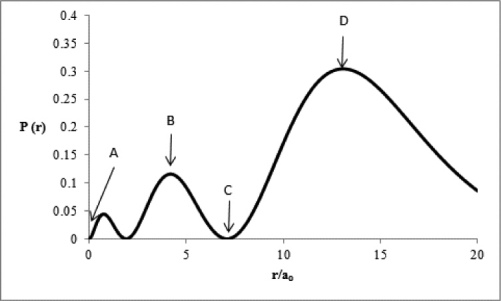 Identify: (1)where the atomic nucleus is located; (2)a radial node; (3)the most probable distance of the electron from the nucleus; and (4)the principal quantum number for this orbital.
Identify: (1)where the atomic nucleus is located; (2)a radial node; (3)the most probable distance of the electron from the nucleus; and (4)the principal quantum number for this orbital.
Definitions:
Q28: What site became a venerated pilgrimage center
Q52: Which of the following regarding Lewis dot
Q67: A 50.0 g piece of iron is
Q110: Which of the following is most likely
Q112: A 75 lb child is to receive
Q114: On average,the total body content of cholesterol
Q120: Which of the following is probably a
Q129: Zinc oxide is found in ointments for
Q142: Indicate which metal requires the shortest
Q146: A Lewis structure of aspirin without the