The energy change for an electronic transition in a one-electron atom or ion (H,He+,Li2+,etc.) from ninitial to nfinal is given by 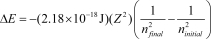 ,where Z is the atomic number. Which one of the following species will have the longest wavelength emission line for the transition between the ninitial = 2 and nfinal = 1 levels?
,where Z is the atomic number. Which one of the following species will have the longest wavelength emission line for the transition between the ninitial = 2 and nfinal = 1 levels?
Definitions:
Technique
A specific method or way of doing something, often involving a particular skill set or procedure.
Component
A part or element of a larger whole, especially a part of a machine or a system.
Autism
A developmental disorder characterized by challenges with social skills, repetitive behaviors, and, in some cases, nonstandard ways of learning, paying attention, or reacting to different sensations.
Social Interactions
Exchanges between two or more individuals, influencing their behaviors and emotions through communication and actions.
Q20: Room temperature is often taken to
Q27: Table sugar (sucrose,C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>)dissolves in water.This process _<br>A)is
Q37: A spare, geometric style of Modern architecture
Q38: Point d in the phase diagram below
Q58: Nuclei with certain numbers of protons and
Q60: Indicate which of the following pairs of
Q73: Briefly explain the following observations using intermolecular
Q88: A graduated cylinder is filled with water
Q100: Calculate the number of hydrogen atoms
Q133: Which of the following bonds will be