The energy of a one-electron atom or ion is given by 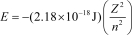 ,where Z is the atomic number of the element.Which of the following one-electron ions would require the most energy to remove its electron?
,where Z is the atomic number of the element.Which of the following one-electron ions would require the most energy to remove its electron?
Definitions:
Fixed Costs
Expenses that do not change with the volume of production or sales, such as rent, salaries, and insurance.
Q15: Carbonyl dihalides (COX<sub>2</sub> with X = I,Cl,or
Q22: What is the shortest wavelength of light
Q33: How does Umberto Boccioni's Unique Forms of
Q62: Label each of the following as either
Q90: Weapons-grade plutonium consists of 93% Pu-239 (239.0522
Q120: Label each of the following as either
Q121: The largest gold bar in the world
Q126: Which characteristic would you expect indium NOT
Q151: Give an example of a nonmetal.
Q164: Which of the following statements is false?<br>A)Ionic