Consider the following Lewis structure: 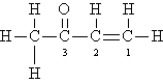 Which statement about the molecule is false?
Which statement about the molecule is false?
Definitions:
Scientific Calculator
A specialized calculator designed to perform complex mathematical functions, used in educational and professional settings.
Customer Benefit
The advantage or value a consumer receives from purchasing and using a product or service, which motivates the buying decision.
Patisserie
A shop or business specializing in the making and selling of pastries, cakes, and other baked goods.
Premium Approach
A marketing strategy that emphasizes the high quality and exclusiveness of a product, targeting consumers willing to pay more.
Q2: What is true about the value
Q17: Girl Gathering Saffron Crocus Flowers (Fig. 4-1)
Q20: From which culture did the architects of
Q27: What was the function of the colossal
Q30: Why did Ashoka erect monuments to Buddha?
Q43: [Ar]4s<sup>1</sup>3d<sup>5</sup>
Q56: What is the kinetic energy of
Q68: Which of the following combinations of quantum
Q86: The change in enthalpy can always be
Q132: The _ quantum number is related to