Consider the following Lewis structure: 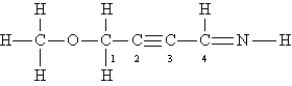 What is the hybridization of the atoms O,C-1,C-2,and C-4?
What is the hybridization of the atoms O,C-1,C-2,and C-4?
Definitions:
Homosexual Orientation
A sexual orientation characterized by a romantic or sexual attraction to individuals of the same gender.
Intimate Relationship
A close and personal connection between individuals, characterized by emotional, physical, or sexual closeness.
Young Female
A girl or young woman in her late childhood to teenage years, experiencing significant developmental, emotional, and physical changes.
Straight
Often used to describe heterosexual individuals, but can also refer to clarity or directness in context.
Q3: How were sculptural features of Chichen Itza
Q8: What method of dating cave paintings and
Q12: In the Icon of Saint Michael the
Q16: What are the basic beliefs of the
Q18: Which style did the artists use to
Q19: Which of these statements about benzene
Q20: How would you compare the artistic representations
Q27: Why does the date for the transition
Q74: Which of the following molecules has a
Q105: The molecular structure of BrF<sub>6</sub><sup>+</sup> is<br>A)pyramidal<br>B)none of