Consider the following Lewis structure: 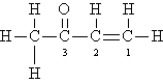 Which statement about the molecule is false?
Which statement about the molecule is false?
Definitions:
Oligopoly
A market structure characterized by a small number of firms controlling a large portion of the market share, leading to limited competition.
Long-run Equilibrium
A state in which all factors of production and costs are variable, and all firms in an industry are making normal profit, resulting in market stability over time.
Average Total Cost
The total cost of production (fixed and variable costs) divided by the total quantity of output produced.
Profit-maximizing Price
The price at which a firm can sell its product to maximize its profit, determined by market demand and production costs.
Q12: Upright stone slabs used in cemeteries as
Q15: What formal elements allow the viewer to
Q17: CO<sub>2</sub><br>A)linear<br>B)trigonal planar<br>C)tetrahedral<br>D)bent<br>E)none of these
Q20: Consider the reaction: 2ClF<sub>3</sub>(g)+ 2NH<sub>3</sub>(g) <span
Q24: How did Chinese art explore human relationships
Q27: Which of the following atoms has the
Q27: Cylinder seals, which were often buried with
Q32: How does Islamic art synthesize a broad
Q52: When an electron pair is shared
Q59: For which of the following diatomic molecules