The H value for the reaction 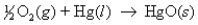 is -90.8 kJ.How much heat is released when 66.9 g Hg is reacted with oxygen?
is -90.8 kJ.How much heat is released when 66.9 g Hg is reacted with oxygen?
Definitions:
Motivation and Emotion
The psychological and physiological states that drive individuals towards actions and influence their feelings and behaviors.
Approaches
Various methods or perspectives used to understand, explain, and study human behavior and mental processes in psychology.
Circular Reasoning
A logical fallacy in which the conclusion is supported by premises that presuppose the conclusion, offering no actual evidence.
Premise
A statement or idea that is taken as the basis for reasoning or argument.
Q2: Stable molecules usually contain atoms that have
Q12: What is the wavelength of light
Q15: A substance,A<sub>2</sub>B,has the composition by mass of
Q43: The standard temperature for gases is<br>A)100°C<br>B)0°C<br>C)32°C<br>D)212°F<br>E)0°F
Q51: Vitamin C contains the elements C,H,and O.It
Q59: Which of the following atoms would have
Q63: Roundup,an herbicide manufactured by Monsanto,has the formula
Q77: Which of the following statements is
Q92: The molar mass of an insecticide,dibromoethane,is 187.9
Q131: To balance a chemical equation,the coefficients must