Given: Cu2O(s) + 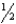 O2(g) 2CuO(s) H° = -144 kJ
O2(g) 2CuO(s) H° = -144 kJ
Cu2O(s) Cu(s) + CuO(s) H° = +11 kJ
Calculate the standard enthalpy of formation of CuO(s) .
Definitions:
Self-Esteem
An individual’s overall subjective emotional evaluation of their own worth.
Competent
Refers to having the necessary ability, knowledge, or skill to perform a task or job successfully.
Extrinsic
Originating from or motivated by external factors outside of the individual's own self, often relating to rewards or outside recognition.
Willingness
The quality or state of being prepared to do something; readiness.
Q20: You have 75.0 mL of a 2.50
Q24: Two metals of equal mass with different
Q25: A 3.60-L sample of carbon monoxide is
Q44: [He]2s<sup>2</sup>2p<sup>3</sup>
Q46: Explain the concept of delocalization of electrons
Q77: Which of the following statements is true?<br>A)The
Q85: What is the enthalpy change when
Q95: Dalton's law of partial pressures states that:<br>A)Equal
Q97: The electron configuration of a particular
Q113: In the gaseous phase,which of the following