Consider the reaction H2(g) + 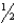 O2(g) H2O(l) H° = -286 kJ
O2(g) H2O(l) H° = -286 kJ
Which of the following is true?
Definitions:
Note
In financial contexts, a note is a legal document representing a loan or a promise to pay a specific sum of money at a future date.
Sells
Involves transferring ownership of a good or service to a buyer for a price.
HDC Status
An abbreviation for Holder in Due Course Status, it refers to a legal shield that provides protection for the possessor of a negotiable instrument from certain defenses if the instrument was acquired in good faith.
Irregularity
A deviation from what is standard, normal, or expected, often indicating a potential issue or problem.
Q7: How did the artists of Lascaux fuel
Q15: A state function does not depend on
Q25: How many structural and geometrical isomers are
Q38: The boiling point of methanol is much
Q45: Which of the following aqueous solutions contains
Q81: Of energy,work,enthalpy,and heat,how many are state functions?<br>A)0<br>B)1<br>C)2<br>D)3<br>E)4
Q81: The ratio of the number of moles
Q107: What volume of carbon dioxide measured at
Q108: Identify the type of organic compound shown:
Q114: A 3.00-g sample of an alloy (containing