The heat of formation of Fe2O3(s) is -826.0 kJ/mol.Calculate the heat of the reaction 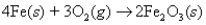 when a 53.99-g sample of iron is reacted.
when a 53.99-g sample of iron is reacted.
Definitions:
De Facto
A term used to describe practices or conditions that exist in reality, even if they are not officially recognized by laws.
Incorporators' Addresses
The physical or mailing addresses of the individuals who file the articles of incorporation, legally founding a corporation.
Defective Corporation
A corporation that has not completely complied with all legal requirements for incorporation, but may still operate as a corporation under certain conditions.
Shareholder Manager
A shareholder who also plays a direct role in the management of a company.
Q5: The molecular structure of XeF<sub>5</sub><sup>+</sup> is<br>A)trigonal bipyramidal<br>B)square
Q15: The compound below is the carbon skeleton
Q26: A fuel-air mixture is placed in a
Q31: How many nonbonded electron pairs are in
Q78: What is true about the value of
Q79: What is the coefficient for oxygen
Q96: In the molecule XeF<sub>2</sub>,how many pairs of
Q103: In general,the ionization energy and electron affinity
Q119: Diffraction results when light is scattered from
Q143: Which of the following ionic compounds has