A mixture of KCl and KClO3 weighing 1.34 grams was heated;the dry O2 generated occupied 143 mL at STP.What percent of the original mixture was KClO3,which decomposes as follows: 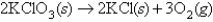
Definitions:
Franchisor
The owner of the trade name or trademark in a franchise.
Franchisee
An individual or company that is granted the right by a franchisor to conduct business under an established brand's trademark and business model.
Trade Agreements
Contracts between nations that determine the terms of trade, including tariffs, duties, and quotas, to facilitate international buying and selling of goods and services.
General Personal Jurisdiction
A doctrine permitting adjudication of any claims against a defendant regardless of whether the claim has anything to do with the forum.
Q2: When 0.72 g of a liquid is
Q38: What mass of calcium chloride,CaCl<sub>2</sub>,is needed to
Q41: A 7.11-g sample of potassium chlorate
Q74: Which of the following has the smallest
Q81: The phenomenon called _ contraction is responsible
Q85: A 38.1-g sample of SrCl<sub>2</sub> is dissolved
Q88: Which one of the following statements
Q97: Aqueous solutions of potassium sulfate and ammonium
Q104: [Ar]4s<sup>1</sup>3d<sup>10</sup>
Q138: Which of the following molecules is non-polar