Given the equation: 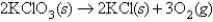 A 3.00-g sample of KClO3 is decomposed and the oxygen at 24.0°C and 0.717 atm is collected.What volume of oxygen gas will be collected assuming 100% yield?
A 3.00-g sample of KClO3 is decomposed and the oxygen at 24.0°C and 0.717 atm is collected.What volume of oxygen gas will be collected assuming 100% yield?
Definitions:
Manufacturing Business
An enterprise engaged in the industrial production of goods from raw materials through the use of labor, machinery, and equipment.
Men's Clothing Store
A retail establishment specializing in clothing for men, offering a variety of apparel and accessories.
Retail Business
A commercial strategy where products are sold directly to end-users, usually in small amounts.
Legal Taxable Entity
An organization or individual recognized by law as a unit that is required to pay taxes on income, assets, or transactions.
Q12: What volume is occupied by 21.0 g
Q15: An atom of fluorine contains nine electrons.How
Q20: A room is 16 ft
Q48: Which of the following will increase the
Q59: A mixture is prepared from 15.0 L
Q77: A molecule that has a center of
Q80: The net ionic equation contains which of
Q81: Copper exhibits the expected electron configuration.
Q109: A 1.59-g sample of a metal chloride,MCl<sub>2</sub>,is
Q149: Which factor is not characteristic of a