Reaction of methane with oxygen really proceeds in two steps: 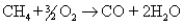
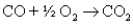 A sample of CH4 is burned in an excess of O2 to give 3.0 moles of H2O.How many moles of CH4 were in the original sample?
A sample of CH4 is burned in an excess of O2 to give 3.0 moles of H2O.How many moles of CH4 were in the original sample?
Definitions:
Truthful
Truthful means being honest or accurate, not hiding the reality of a situation or information from those who are entitled to know.
Aging Men
Refers to the natural process and implications of becoming older, specifically in the context of male individuals.
Ethical Danger
Situations or actions that pose a risk of compromising ethical standards, leading to decisions or behaviors that are morally questionable.
Agents
Agents in negotiations refer to individuals who represent another party's interests, acting on their behalf to achieve favorable terms in a deal or agreement.
Q7: If 45.0 g of O<sub>2</sub> are mixed
Q18: An example of a secondary structure of
Q21: Choose the correct molecular structure for SeBr<sub>4</sub>.<br>A)trigonal
Q25: How many structural and geometrical isomers are
Q62: Which of the following pairs is incorrect?<br>A)sucrose
Q70: At the same temperature,lighter molecules have a
Q82: Which metal is most widely used in
Q88: Which one of the following statements
Q111: Which of the following salts is insoluble
Q128: Naturally occurring element X exists in three