Consider the following reaction: 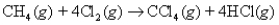 What mass of CCl4 is formed by the reaction of 5.14 g of methane with an excess of chlorine?
What mass of CCl4 is formed by the reaction of 5.14 g of methane with an excess of chlorine?
Definitions:
B2B Commerce
Business-to-Business commerce, wherein transactions are conducted between companies rather than between companies and consumers.
Bookmark Feature
A functionality that allows users to mark specific points or pages in digital documents or web browsers for quick access in the future.
Internet-Connected Computer
A computer that is capable of sending and receiving data through internet networks, enabling access to the worldwide web and other online services.
M-Commerce
Mobile commerce, the buying and selling of goods and services through wireless handheld devices like smartphones and tablets.
Q17: Balance the following equation: KI +
Q28: Order the following bonds from highest to
Q31: The correct coefficients to balance the
Q37: Name the following: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Name the
Q43: Alkaline earth metals react less vigorously with
Q84: Indicate the total number of isomers in
Q94: Choose the correct molecular structure for ClO<sub>2</sub><sup>-</sup>.<br>A)trigonal
Q103: The complex ion Co(NH<sub>3</sub>)<sub>6</sub><sup>2+</sup> (three unpaired electrons)is
Q117: Soluble ionic compounds containing the hydroxide ion
Q143: _ results when light is scattered from