The following two reactions are important in the blast furnace production of iron metal from iron ore (Fe2O3) : 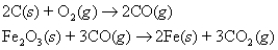 Using these balanced reactions,how many moles of O2 are required for the production of 3.19 kg of Fe?
Using these balanced reactions,how many moles of O2 are required for the production of 3.19 kg of Fe?
Definitions:
Net Method
An accounting method for recording purchases where the purchase price includes a cash discount for early payment.
Periodic Inventory System
An accounting method where inventory is physically counted and valued at set intervals, affecting the cost of goods sold calculation.
Discounts Lost
Refers to the additional cost a company incurs for not taking advantage of the cash discounts offered by suppliers for early payments.
Net Method
The Net Method is an accounting procedure that records the purchase of goods or services after deducting any discounts for early payment.
Q32: Calculate the root mean square velocity for
Q37: Name the following: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="Name the
Q59: Choose the metal with the largest first
Q75: According to the first law of
Q86: Aspirin is formed via a(n)_ reaction.<br>A)combustion<br>B)hydrogenation<br>C)addition<br>D)condensation<br>E)substitution
Q89: How many unpaired electrons are there in
Q106: Order the elements S,Cl,and F in terms
Q133: The most important hydride of nitrogen is<br>A)ammonia<br>B)hydrazine<br>C)styrofoam<br>D)agricultural
Q142: The strongest reducing agent in the alkali
Q163: An anticodon<br>A)is part of tRNA<br>B)complements the codon