The following equation describes the oxidation of ethanol to acetic acid by potassium permanganate: 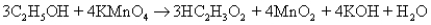 5.00 g of ethanol and an excess of aqueous KMnO4 are reacted,and 4.24 g of HC2H3O2 result.What is the percent yield?
5.00 g of ethanol and an excess of aqueous KMnO4 are reacted,and 4.24 g of HC2H3O2 result.What is the percent yield?
Definitions:
Friendship Patterns
The recurrent behaviors and interactions that characterize the relationships between friends, often influenced by social, cultural, and individual factors.
Apartment Buildings
Multi-unit residential buildings where individuals or families live in separate apartments, but share common areas such as hallways and exterior spaces.
Proximity
The physical closeness between people, objects, or geographical locations, which can influence relationships and interactions.
Attitudes
Psychological tendencies expressed by evaluating an entity with some degree of favor or disfavor.
Q10: What ions are very important for the
Q46: Consider an aqueous solution of calcium nitrate
Q56: What is the kinetic energy of
Q58: Why is nitrogen not able to form
Q79: The complex ion Ni(NH<sub>3</sub>)<sub>6</sub><sup>2+</sup> (two unpaired electrons)is
Q80: An oxide of iron has the formula
Q116: Polystyrene is an addition polymer of styrene.What
Q121: Choose the metal with the smallest radius.<br>A)Ca<br>B)Na<br>C)K<br>D)Mg<br>E)Al
Q126: In the balanced equation for the
Q139: What nitrogen-containing compound is used as rocket