Consider the following reaction: 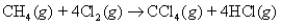 What mass of CCl4 will be formed if 1.20 moles of methane react with 1.11 moles of chlorine?
What mass of CCl4 will be formed if 1.20 moles of methane react with 1.11 moles of chlorine?
Definitions:
Emotional Instability
A characteristic of experiencing rapid and intense emotional changes or mood swings.
Dispositional Contempt
A habitual feeling of disdain or scorn towards others or their actions.
High Self-Monitoring
A personality trait that refers to an individual's ability to adjust their behavior to fit the current situation.
Speed Dates
Short, timed meetings arranged between individuals seeking romantic partners, allowing both parties to quickly gauge potential interest.
Q9: I
Q20: You have 75.0 mL of a 2.50
Q48: Which of the following will increase the
Q76: FeCl<sub>3</sub>(aq)+ Na<sub>2</sub>SO<sub>4</sub>(aq)
Q78: In which flask are the molecules least
Q91: Which of the following is known as
Q99: The molar mass of the compound formed
Q133: Define stereoisomerism.
Q139: What nitrogen-containing compound is used as rocket
Q149: Choose the correct molecular structure for XeF<sub>4</sub>.<br>A)trigonal